Which of the Following Shows the Combustion of a Hydrocarbon
A 2 1 2. As the molecular mass of hydrocarbon increases it starts burning with a yellow coloured flame showing incomplete burning.

Combustion Of Hydrocarbons Hydrocarbon Combustion Products
FeedbackThe correct answer is.

. Which of the following shows the combustion of a hydrocarbon. Always contains a three carbon ring. Problem 46 Hard Difficulty.
C Nonane is a solid at room temperature. It inculcates necessary insights on Hydrocarbon topic that students have to know in order to excel in CBSE Class 11 final examination and graduate entrance. A combustion reaction is a major class of chemical reactions commonly referred to as burning In the most general sense combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product.
Propene C3H6 is a hydrocarbon which is the term used to designate a compound that consists of only carbon and hydrogen. D Nonane does not undergo combustion. Moreover it is now known that the KPg soot and PAHs reveal a signature consistent with hydrocarbon combustion at the impact site Belcher et al 2005 2009.
BALANCING EQUATIONS FOR HYDROCARBON COMBUSTION CxHy 02 CO2 H20 where x and y are numbers Balance the following equations by writing in the simplest whole number coefficients in the spaces provided. E 2 2 2. The products from the combustion of hydrocarbon fuels can be identified with the following set up in the lab.
We review their content and use your feedback to keep the quality high. BALANCING EQUATIONS FOR HYDROCARBON bartleby. Y refers to the number of hydrogen atoms in the hydrocarbon.
Hexane was burnt in a crucible and incomplete combustion occurred. NCERT Exemplar Chemistry Class 11 Chapter 13 Hydrocarbons will guide you in understanding the topic from the exam point of view. The species are CO 2 H 2 O and N 2 with mole fractions of 133 127 and 740 respectively and the temperature is 2867 K calculated by CEA.
NCERT Exemplar Solutions Class 11 Chemistry Chapter 13 Free PDF Download. Preview 15 questions Show answers Question 1. Orsat analysis of combustion of hydrocarbon fuels shows 120 CO2 10 02 and the balance N2.
Hydrocarbons react with oxygen to form carbon dioxide and water. 2C2H2 5O2 4CO2 2H2O. Choose the three numbers that in the correct order balance the following incomplete combustion reaction.
A Nonane is soluble in water. To simulate the supersonic environment in the combustor the pressures on the inlet and outlet boundary of the. Calculate the excess air 1.
To play this quiz please finish editing it. View the full answer. Which equation represents incomplete combustion.
For a hydrocarbon if complete ignition occurs then it burns with a blue flame. An Indonesian Coal has the following Ultimate analysis Parameter Moisture Ash Carbon Hydrogen Nitrogen Indonesian Coal 943 1399 5896 416 102 056 1188 8 ka Calculate the Orsat Analysis of the gas for. Harvey et al 2008 see also PAH abundance curve on Figure 164.
CH2CHCH3 O2 c. D 4 2 4. This tells you that all the carbon that was a part of the hydrocarbon will now be a part of the carbon.
E Nonane floats on the surface of water. In the context of increasing the energy utilization of hastily depleting conventional hydrocarbon based fuels and regulating the associated emissions the present experimental work investigates the conjoint effect of partially cooled exhaust gas recirculation on magnetic field-assisted combustion of gasoline in a multicylinder MPFI spark ignition engine. Contains -CH₃ groups joined by single bonds.
Ethane C 2H 6 oxygen Æ 3. It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Complete and balance each hydrocarbon combustion reaction.
Complete combustion of a sample of a hydrocarbon in the presence of excess oxygen produces equal molar quantities of carbon dioxide and water. It releases the maximum amount of energy and produces carbon dioxide and water. As hydrocarbon combustion will produce carbon dioxide water and energy so correct equation is CxHy O2 --- CO2 H2O energy so correct answer is opiton A Answer part II As per the reaction AgNO3 NaI -- NaNO3 AgI so one mole of AgNO3.
Which of the following could be the molecular formula of the compound. Combustion Reactions We will focus on the combustion of hydrocarbons. CH3CH2CH2CH3 O2 b.
According to the IUPAC convention for chemical naming which part of. Methane CH 4 oxygen Æ 2. Oxygen-Take a splinter and take it near near the test tubeIf oxygen is released then the splinter will burn brightly and with a pop sound.
C 2 2 4. N refers to the number of oxygen atoms required in the hydrocarbon combustion reaction. Watch this demonstation of identifying the products of combustion of hydrocarbons.
Know more about Pyrolysis of Alkanes. Has two fewer carbon atoms than the corresponding alkane. Butane C 4H 10.
The lack of evidence for wildfires along with the high abundance of non-charred plant remains found in non-marine KPg boundary impact rocks. C8H16 I know the answer is C2H6 but I need it explained. C H O C O C O H O 3 8 2 2 2 𝑥 𝑦 𝑧.
B Nonane is a gas at room temperature. Good signs that youre. This quiz is incomplete.
Has two fewer hydrogen atoms than the corresponding alkane. 1l 2 C2H6 etháne C3H8 propane С4Н10 butane b H20 6 3 7 02. Propane C 3H 8 oxygen Æ 4.
When a hydrocarbon undergoes complete combustion only two products are formed. B 4 4 2. The main flow is simulated with the products of complete combustion of kerosene at a high temperature.
Fuel occurs when there is a good supply of oxygen. The burning of hydrocarbons becomes difficult with increasing molecular weights. C x H y N O 2 x C O 2 y 2 H 2 O x refers to the number of carbon atoms in the hydrocarbon.
Has two fewer hydrogen atoms than the corresponding alkane. Which of the following is true of nonane C₉H₂₀ which has a density of 079 gmL melts at -51 C and boils at 151 C. A Hydrocarbons are the compound of hydrogen and carbon as it is already cleared by the name HYDROCARBONSo after combustion CO2 carbon dioxide and H2O water.

Combustion Reaction Definition And Examples Reactions Chemical Reactions Chemical Bond

Cutaway Drawing Of Single Cylinder Air Cooled Spark Ignition Engine Cylinder Engine Types Air
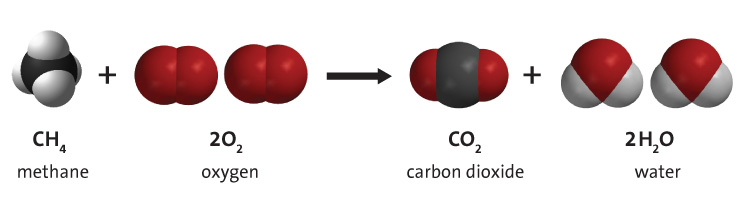
Hydrocarbon Combustion Energy Education

Pin On Ideas To Use For Other Reasons

Complete Combustion Of Methane Ch4 Balanced Equation Youtube

Rocket Forces Force Rocket Learning Science

Ocr Gateway C1 Complete And Incomplete Combustion Higher Completed Equations Tutorial
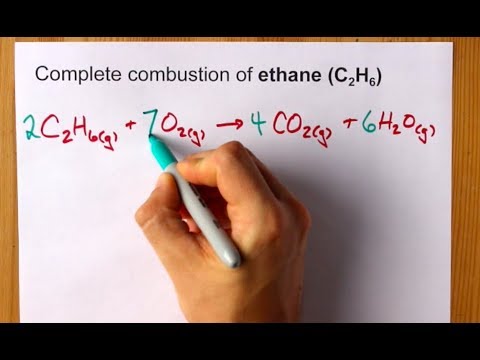
Complete Combustion Of Ethane C2h6 Balanced Equation Youtube

Case Based Mcq Chemistry In Automobiles For An Internal Combustion

Complete Combustion Of Methane Ch4 Balanced Equation Youtube

Noticeable Symptoms Of A Bad Valve Stem Seal Repair Auto Repair Repair And Maintenance
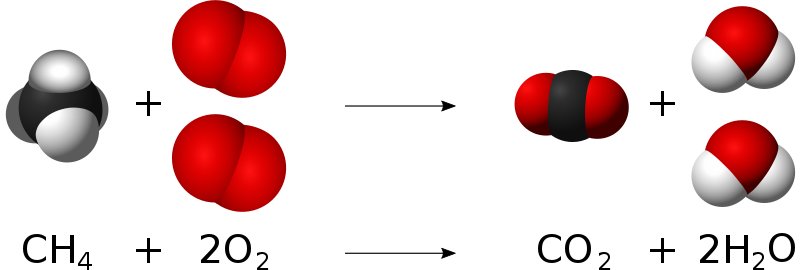
Combustion Simple English Wikipedia The Free Encyclopedia

Things To Know About The Important Components Of The Emission Control System Classic Cars Repair Best Gas Mileage

Complete Combustion Of Hexane C6h14 Balanced Equation Youtube

Hydrocarbons Advanced Scribble Notes Teaching Chemistry High School Chemistry Organic Chemistry Study

1994 Toyota Pickup Vacuum Hose Diagram 7 4runner Toyota Diagram

Pin By Sanghita Dey On Cbse Class 10 In 2021 Physical Properties Pure Products Sour Taste

Pin Auf Engine Types And Their Operation

Complete Combustion Of Propane C3h8 Balanced Equation Youtube
Comments
Post a Comment